CADMIUM BIOSORPTION RATE IN PROTONATED
SARGASSUM BIOMASS  Abbreviated version of a contribution shortly to be published in
ENVIRONMENTAL SCIENCE & TECHNOLOGY (1998)
Jinbai
Yang and Bohumil
Volesky1
Department of Chemical Engineering , McGill University,
3610 University Street, Montreal,
Quebec, Canada H3A 2B2
Abbreviated version of a contribution shortly to be published in
ENVIRONMENTAL SCIENCE & TECHNOLOGY (1998)
Jinbai
Yang and Bohumil
Volesky1
Department of Chemical Engineering , McGill University,
3610 University Street, Montreal,
Quebec, Canada H3A 2B2
Abstract
Dynamic biosorption rate
of the heavy metal ion Cd2+ onto protonated non-living brown
alga Sargassum fluitans biomass was determined at constant pH 4.0.
An one dimensional intraparticle diffusion model, combined with Langmuir
sorption isotherm assumption, was developed to describe the overall biosorption
rate of cadmium ions in flat seaweed biomass particles. The model equations
were solved numerically yielding the effective diffusion coefficient De
about 3.5x10-6 cm2/sec, which is of the same order
of magnitude as the molecular diffusion coefficient for cadmium ions in
aqueous solution.
Introduction
Biosorption of the non-living
brown alga Sargassum fluitans has been found particularly effective
in binding metal ions of cadmium. High sorption capacity (exceeding 100
mg/g), easy regeneration and low costs make this biomass of special interest
for the purification of high volumes of low-concentration wastewater (1,
2, 3). For any practical applications, the sorption process rate and
the dynamic behavior of the system are very important factors. While several
mechanisms have been proposed to describe the rate of the biosorption process
(4, 5, 6), the resulting diffusion coefficients often did not yield
reasonable values. In our earlier work (7), an intraparticle diffusion
model was proposed for the acidic desorption process for the Sargassum
fluitans biomass. The effective diffusion coefficient of Cd2+
ions was determined in the range of 0.23 - 0.55 of the molecular diffusion
coefficient. The present work investigated the sorption behavior of protonated
Sargassum biomass at an optimum sorption pH 4.0 directly. A one-dimensional
plate diffusion model could enable the prediction of the dynamic behavior
of the metal ion binding process.
Material and Methods
Dry Sargassum fluitans
particles of the same thickness (0.1 mm) were cross-linked and protonated
with formaldehyde and HCl according to Leusch et al. (1). Unless
specified otherwise, the biomass particle size used was (1.0 - 1.4 ) mm.
An end-point titration process
was used to measure the biosorption rate. A certain amount of biomass
and Cd(NO3)2 solution were stirred while the pH of
the solution was maintained (NaOH addition recorded) by a computer-driven
autotitrator assembly in the end-point mode. The solution was sampled at
pre-defined time intervals and analyzed for metal concentration (AAS).
The metal binding was determined
by the simple mass balance q=(C0 - Cb)
V / W.
Experimental Results
Sorption rate.
As external mass transfer resistance was eliminated by proper agitation
(3 Hz ), the biosorption rate of the Cd cation onto the (1.0-1.4 mm) biomass
particles could be established from Figure 1.
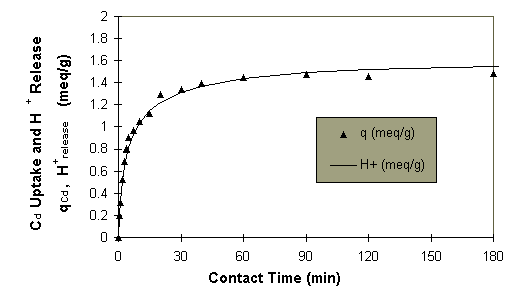 Figure 1: Dynamics of the cadmium metal uptake and proton
release in time at pH4.0.
Figure 1: Dynamics of the cadmium metal uptake and proton
release in time at pH4.0.
The first stage of sorption
featured a high rate, about 75% of the total cadmium adsorption took place
in 15 min. The second stage was slower, taking approximately 3 hours to
reach the sorption equilibrium. The rate of the proton released reflected
the cadmium ion uptake rate well throughout the process. The proton release
was calculated from the consumption of the NaOH added to the sorption system
for maintaining the constant pH 4.0. Similar results were obtained for
other sizes of biomass particles.
The effect of biosorbent
particle size on the Cd sorption rate. The time profiles of the dimensionless
Cd concentration for different sizes of Sargassum biosorbent particles
were similar (Figure 2).
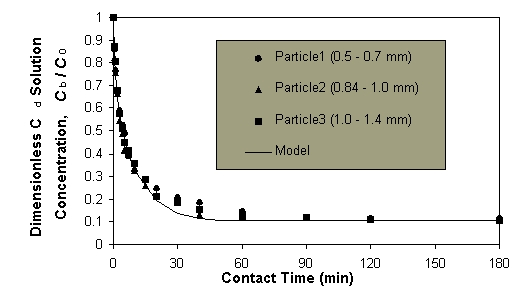 Figure 2: The equilibrium Cd concentration in solution for
different particle sizes.
Figure 2: The equilibrium Cd concentration in solution for
different particle sizes.
The solid curve is the model
prediction (described later) and the scattered points are from the experiments.
The concentration profiles for three different particle sizes of (0.5 -
0.7) mm, (0.84-1.00) mm and (1.0-1.4) mm agreed with each other within
the deviation of about 5%. This indicates that the overall sorption rate
of Cd by the biomass is actually practically independent of the biosorbent
particle size used.
The effect of cross-linking
on the Cd sorption rate. The sorption rate for the cross-linked biomass
was significantly lower than that for native biomass (Figure 3).
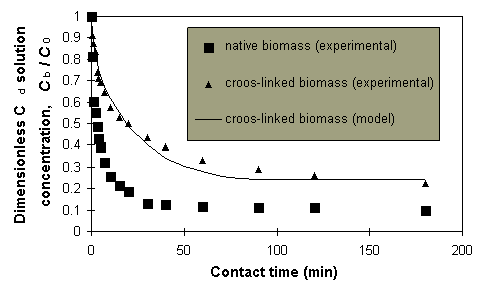 Figure 3: Cd concentration time profiles for cross-linked
and not cross-linked biomass.
Figure 3: Cd concentration time profiles for cross-linked
and not cross-linked biomass.
Mathematical Model
For quantitative description
of the biosorption process dynamics, the similar assumptions as in our
earlier work on cadmium desorption(7)are made here as follows:
-
The particle-to-liquid mass transfer resistance has been eliminated through
adequate turbulence created by proper agitation. and the overall sorption
rate is controlled by the diffusion of the Cd cation inside the biomass
material.
-
The intraparticle diffusion inside the biomass is one dimensional, i.e.
in the thickwise direction. Because the thickness of the relatively flat
seaweed Sargassum biomass particle is much smaller than the length
and width, the biomass particle can be considered as a thin plate.
-
The amount of metal ions adsorbed inside the biomass particle is in equilibrium
with the metal concentration in the liquid phase and the Langmuir sorption
isotherm relationship holds:
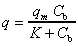 (1)
Based on the model assumptions,
the mass conservation equations for metal ions in the biomass particle
and bulk solution are as follows:
(1)
Based on the model assumptions,
the mass conservation equations for metal ions in the biomass particle
and bulk solution are as follows:
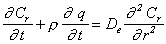 (2)
(2)
 (3)
Please see the Glossary
at the end of text for symbols. De represents the effective
intraparticle diffusion coefficient.
(3)
Please see the Glossary
at the end of text for symbols. De represents the effective
intraparticle diffusion coefficient.
The boundary and initial
conditions for the sorption process are as follows:
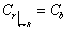 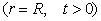 (4)
(4)
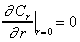 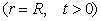 (5)
(5)
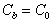 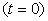 (6)
(6)
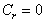 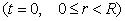 (7)
Equations (2) and (3) are he
simultaneous non-linear partial differential equations (PDEs) with respect
to Cr and Cb, respectively.
The Galerkin Finite Element Method (GFEM) (8) and the Euler backward
integration in time was applied to obtain the numerical solution.
(7)
Equations (2) and (3) are he
simultaneous non-linear partial differential equations (PDEs) with respect
to Cr and Cb, respectively.
The Galerkin Finite Element Method (GFEM) (8) and the Euler backward
integration in time was applied to obtain the numerical solution.
The intraparticle diffusion
coefficient De could be regressed from the comparison
of the simulated profile curves and the experimental results by minimizing
the following objective function:
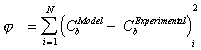 (8)
where i is the ith experimental data point
and N is the total number of experimental data points.
(8)
where i is the ith experimental data point
and N is the total number of experimental data points.
Equilibrium biosorption
experiments were conducted at pH 4.0 for regression of the Langmuir parameters
in the model equations. The regressed parameters are K = 0.035 mmol/L
and qm = 0.994 mmol/g, respectively. The model-simulated
concentration profile curve for protonated biomass and cross-linked biomass
were plotted as a solid line in Figure 2 and Figure 3, respectively. The
diffusion coefficient De values regressed from the corresponding
experimental data are 3.5x10-6 cm2/sec and 1.0x10-6
cm2/sec, respectively. The fact that the simulation curve and
the experimental data points are in a good agreement (within 10% average
deviation) demonstrates that the model can describe the experimental data
with an acceptable accuracy.
Discussion
The metal uptake and
the proton release rate. Biosorption of Cd took place very fast
during the initial stage of contact (Figure 1). The agreement between the
cadmium metal uptake and the proton release during the sorption process
indicates an equivalent ion exchange between Cd2+ and H+.
With a fast pH probe response and titration speed, the computer-recorded
titration volume of the NaOH vs. contact time correlation could
be used to regress the effective diffusion coefficient directly. This method
provides a useful alternative to the fast evaluation of the sorption rate,
avoiding the physical difficulty of sampling the reaction solution at the
early stage of the sorption process and the tedious procedure for metal
concentration analysis.
As indicated in Figure 1,
the sorption rate could be divided into two stages, the fast initial rate
followed by a much slower sorption rate. This has often been reported by
other researchers. Brassard et al. (9) attributed the fast initial
metal sorption rate to the surface binding by natural particles and the
following slower sorption to the interior penetration. In the case of Sargassum
seaweed particles, the active binding groups reside in the cell wall
and due to its large surface the initial sorption rate is accelerated.
The actual mechanism of Cd sequestration has been studied only to a limited
extent (10) and should be studied further.
Effect of particle size
on the sorption rate. The experimental results indicated that the particle
size does not affect the sorption rate. While this seems to be contradictory
to the general idea of intraparticle diffusion controlling the process,
it is necessary to point out that the grinding of biomass and particle
size grading by standard sieves only work on the length and width dimensions.
All sizes of the Sargassum biomass chip-like particles are actually
of the same thickness which determines the diffusion distance. This is
reflected in the present model which assumed a one-dimensional thin plate
as the intraparticle diffusion field. A special care was taken in producing
the biomass particles. The structure of the seaweed biomass is not homogeneous
and different parts of the seaweed offer different resistance to grinding.
Thus different sizes of the sieved biomass may differ in composition resulting
in a dependence of the sorption rate on the particle size.
Evaluation of the diffusion
coefficient. In general, the diffusion process inside a porous material
is slower than that in a corresponding homogeneous system having the same
liquid composition as in the pore phase (11, 12). In the brown alga
Sargassum biomass, alginate is the main component responsible for
the metal sorption (13). It is present in a gel form in the cell
wall which appears very porous and easily permeable to small ionic species
(14, 15). The actual mobility of the diffusing entity in the dense-phase
gel may be somewhat reduced by mechanical friction or interaction with
the cell wall molecules. As a result, the calculated intraparticle diffusion
coefficient is an effective diffusion coefficient, i.e. De,
and it is usually smaller than the molecular diffusion coefficient Dm
considered in the absence of the sorbent material matrix. For Cd2+,
Dm was assessed as 7.19x10-6 cm2/sec
(16, 17) and the De /Dm calculated
with the presently regressed value was 0.486 which is in a good agreement
with the one determined for the desorption process (7). A smaller
De /Dm ratio of 0.14, calculated
from the results in Figure 3 for cross-linked biomass, correctly reflects
the retarding effect of cross-linking of the sorbent framework on the sorption
rate.
In summary, the end-point
titration method is suitable for the determination of sorption rate at
a constant pH value. The rate of Cd2+ biosorption on Sargassum
fluitans biomass could be described properly by a simple one-dimensional
intraparticle diffusion model. The diffusion coefficient of Cd2+
ion in the biomass regressed from the model at pH 4.0 was about 3.5 x 10
-6 cm2/sec, this being of the same order of magnitude
as the corresponding molecular diffusion coefficient.
Glossary
C0 , Cb
initial and instant Cd concentration in bulk solution (mg/L)
Cr Cd concentration in bulk solution
at position r and time t (mg/L)
De effective intraparticle diffusion
coefficient (cm2/sec)
Dm molecular diffusion coefficient (cm2/sec)
i is the ith experimental
data
K Langmuir equilibrium constant (mg/L)
N is the total number of experimental data
points.
qm Langmuir maximum uptake (mg/g)
q uptake (mg/g)
r arbitrary position coordinate (cm)
R half thickness of the biomass particle (cm)
St total surface area of biomass
particles (cm2)
t time (sec)
V solution volume (L)
W biomass weight (g)
r density of biomass g/(1000
cm3)
j objective function for
curve fitting.
Literature Cited
(1) Leusch, A.; Holan, Z.R.; Volesky, B. J. Chem. Tech. Biotechnol.
1995,
62, 279-288.
(2) Aldor, I.; Fourest, E.; Volesky, B. Can. J. Chem. Eng. 1995,
73, 516-522.
(3) Volesky, B.; Holan, Z.R. Biotechnol. Prog. 1995,
11, 235-250.
(4) Jang, L.K.; Brand, W.; Resong, M.; Mainieri, W.; Geesey, G.G.
Environ. Prog. 1990,
9, 269-274.
(5) Chen, D.; Lewandowski, Z.; Roe, F.; Surapaneni, P.
Biotechnol. Bioeng.
1993, 41, 755-760.
(6) Apel, M.L.; Torma, A.E. Can. J. Chem. Eng. 1993,
71, 652-656.
(7) Yang, J.; Volesky, B. J. Chem. Technol. Biotechnol. 1996,
66, 355-364.
(8) Lapidus, L.; Pinder, G.E. Numerical Solution of Partial Differential
Equations
in Science and Engineering, Wiley: New York, 1982, pp
(9) Brassard, P.; Macedo, E.; Fish, S. E.T.S. 1996, 30,
3216-3222.
(10) Crist, R.H.; Oberholser, K.; Schwartz, D.; Marzoff, J.; Ryder,
D.; Crist, D.R.
Environ.
Sci. Technol. 1988, 22, 755-760.
(11) Helfferich, F. Ion Exchange, McGraw-Hill: NY, 1962,
pp 299-319.
(12) Westrin, B.; Axelsson, A. Biotechnol. Bioeng. 1991,
38, 439-446.
(13) Fourest, E.; Volesky, B. Appl Biochem Biotechnol 1997,
67, 33-44.
(14) Percival, E.; McDowell, R.H. Chemistry and Enzymology of Marine
Algal
Polysaccharides,
Academic Press: London, U.K., 1967, pp 99-126.
(15) Dodge, J.D. The Fine Structure of Algal Cells, Academic
Press:
London, U.K., 1973, pp 14-45.
(16) Horvath, A.L. Handbook of Aqueous Electrolyte Solutions,
Ellis Horwood: West Sussex, UK, 1985, pp 289.
(17) Dobos, D. A Handbook for Electrochemists in Industry and Universities,
Elsevier Scientific: Amsterdam, The Netherlands, 1994, pp 88.
[ Bact to the Beginning ] [
Bact to the Index ] [Biosorption
Home] [ Dr. Volesky's Home ] |



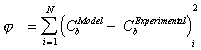 (8)
(8)