Hui Niu
, Bohumil Volesky
* and Newton C. M. Gomesa
http://www.mcgill.ca/biosorption/biosorption.htm
Department of Chemical Engineering , McGill University
*e-mail: boya@chemeng.Lan.mcgill.ca
3610 University Street, MONTREAL, Canada H3A 2B2
*corresponding author
aInstituto de Microbiologia Prof. Paulo de Goes, CCS
Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil
The presence of L-cysteine increased gold-cyanide
biosorption by protonated Bacillus subtilis, Penicillium chrysogenum
and Sargassum fluitans biomass at pH 2 by (148 ~ 250)%. The respective
Au uptake by these biomass types was 20.5 mmol/g
,14.2 mmol/g and 4.7 mmol/g
of Au. Au-loaded biomass can be eluted with 0.1M NaOH. The elution efficiencies
exceeded 90% at pH 5.0 with the Solid-to-Liquid ratio S/L = 4. Biosorption
of anionic AuCN2- complex involved ionizable protonated
cysteine-loaded biomass functional groups. An adverse effect of increasing
solution ionic strength (NaNO3) was explained by a triple layer
surface complexation mechanism. The NO3- anion competed
with AuCN2-. Results confirmed that certain waste
microbial biomaterials are capable of effectively removing and concentrating
gold from solutions containing residual cyanide.
1. INTRODUCTION
Recent experimental results demonstrated that Bacillus
subtilis, Penicillium chrysogenum and Sargassum fluitans
biomass could extract Au from cyanide solution [1]. The main mechanism
of Au biosorption involved anionic AuCN2- species
adsorption onto N-, P-, or O- containing functional groups on biomass through
ion-pairing (H+.- AuCN2-). However, the
capacities for Au biosorption by Bacillus subtilis, Penicillium
chrysogenum and Sargassum fluitans biomass were not encouraging
.
Proteins are known to be capable of complexing with
metal ions. Cysteine, which figures prominently in discussions of metal
ion binding to proteins, has three possible coordination sites, namely
sulfhydryl, amino and carboxylate groups [2]. Hussain attributed the protection
of isolated human lymphocytes from silver toxicity to cysteine through
the formation of Ag-thiol complexes [3]. The complexation of Cu-cysteine
was ascribed to the complexing of Cu to thiol as well as amino groups [4].
These results showed that cysteine had a tendency to combine well with
metals. However, the behavior of L-cysteine in Au-cyanide complex biosorption
have never been examined.
The objectives of this work are to investigate the
effect of L-cysteine on Au biosorption from cyanide solution by dead Bacillus
subtilis, Penicillium chrysogenum and Sargassum fluitans
biomass. The mechanism of Au-cyanide biosorption under these unconventional
conditions was also examined.
2. MATERIALS AND METHODS
2.1 Biosorbent preparation
Waste industrial biomass samples of Bacillus
subtilis and Penicillium chrysogenum were collected from Sichuan
Pharmaceutical Company, Chengdu, P. R. China. Sargassum fluitans
seaweed biomass was collected beach-dried on the Gulf Coast of Florida.
Biomass was ground into particles around (0.5-0.85) mm in diameter, then
washed with 0.2N HNO3 for 4 hrs and rinsed with distilled water
to pH~4.5. Finally, the biomass was dried in the oven at 500C
for 24 hrs to a constant weight.
2.2. Acidification of gold cyanide solution
A solution of AuCN2- was prepared
by dissolving solid NaAuCN 2 in NaOH solution at
pH 11 to simulate the industrial gold cyanide leach solution. Metal biosorption
by biomass is usually taking place at pH values less than pH 6 since some
biomass types or their constituents could seriously hydrolyze at elevated
pH levels [5]. In order to make a full use of the biomass potential to
concentrate gold from the cyanide solution, the pH of the gold cyanide
solution needs to be adjusted for adequate biosorption. Since toxic hydrogen
cyanide gas released at pH lower than 9.3 [6] is extremely dangerous, the
conventional AVR process (Acidification, Volatilization and Reneutralization
of cyanide ) was employed [7]. The only difference from the standard AVR
process in the current experiments was stripping of cyanide gas by nitrogen
instead of air to avoid any oxidation of HCN. Total cyanide analysis was
done by standard cyanide distillation followed by the titrimetric method
for free CN- in the alkaline solution [8].
2.3. Cysteine adsorption by biomass
Approximately 40 mg dried protonated biomass contacted
with 20 ml cysteine solution with certain initial cysteine concentration
0~ 1.2 mmol/l in 150 ml Erlenmeyer flasks. The solution was mixed and left
to equilibrate for 4 hrs. The cysteine uptake was determined from the difference
of cysteine concentrations in the initial and final solutions. Cysteine
was analyzed by a UV-visible spectrophotometer (Cary 1).
2.4. Equilibrium sorption experiments
Approximately 40 mg dried protonated biomass was
combined with 20 ml sodium gold cyanide solution with or without L-cysteine
in 150 ml Erlenmeyer flasks. The solution was gently mixed and equilibrated
for 4 hrs. Uptakes of chromium were determined from the difference of metal
concentrations in the initial and final solutions. The pH of the solutions
before and during the sorption experiments was adjusted with 0.1M NaOH
or HNO3. The ionic strength was controlled by adding NaNO3
. All reagents were ACS reagent grade quality. Au concentration was determined
by a sequential inductively-coupled plasma atomic emission spectrometer
(Thermo Jarrell Ash, Trace Scan).
3. RESULTS AND DISCUSSION
3.1. Effect of L-cysteine on Au biosorption
The effect of L-cysteine on Au biosorption by Bacillus
, Penicillium and Sargassum biomass was examined by varying
L-cysteine concentration in the Au cyanide solution from 0 ~ 0.6 mmol/l
at pH 2.0, with the initial Au concentration of 0.1015 mmol/l. No cyanide
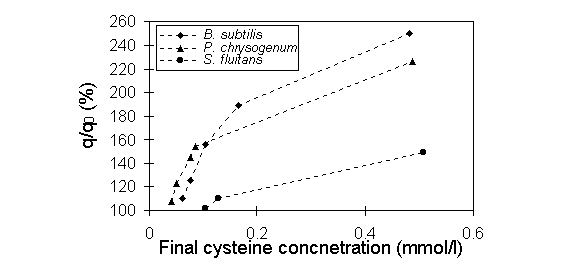
was released during the process. The results are shown in Figure 1. The
final cysteine concentration around 0.5 mmol/l enhanced Au uptakes by Bacillus
, Penicillium and Sargassum biomass up to 250% , 200% and
148%, respectively.
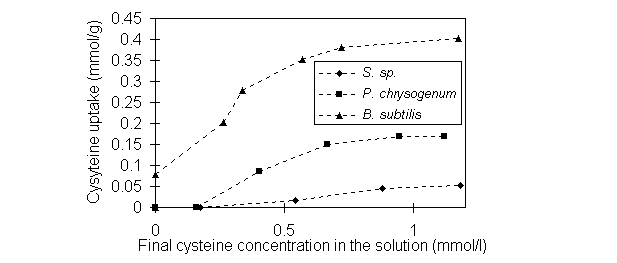
L-cysteine biosorption isotherms for Bacillus
, Penicillium and Sargassum biomass in Figure 2 show encouraging
uptakes by Bacillus and Penicillium biomass, while Sargassum
biomass sorbed very little. Under the experimental conditions, the sequence
for the cysteine uptake by the three biomass types is Bacillus >
Penicillium > Sargassum , which agreed with the sequence of
increased Au uptake in the presence cysteine. Enhancement of Au biosorption
in the presence of cysteine apparently relates to the "bridging" function
provided by cysteine between the Au-cyanide complex and biomass. The main
active sites on the cysteine molecule are sulfhydryl, amino and carboxyl
groups [4]. The dissociation constants (pK) of those groups are respectively
8.12, 10.36 and 1.90 [9]. At low pH 2.0, the carboxyl group is more active
than the other two groups of cysteine and tends to combine with positively
charged groups on biomass.

|
 |
3.2. Effect of pH
The effect of pH on Au biosorption in the presence
of cysteine was examined by varying pH from 2~6. The initial ratio of Au
: cysteine was 1:5. During the process of acidifying the Au cyanide solution
and Au biosorption equilibration, there was no cyanide released from the
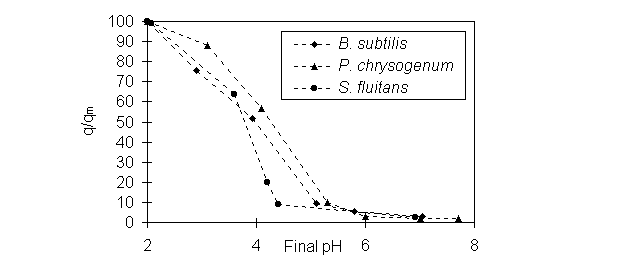
solution. Figure 3 shows that in the presence of cysteine Au adsorption
by Bacillus, Penicillium or Sargassum biomass was
strongly affected by pH. The equilibrium uptakes of Au at pH 2 were greater
than those at pH>2. The pH had a tendency to increase during the equilibration,
hence 0.1 N HNO3 was used to adjust the pH. This observation
is opposite to that reported for biosorption of Zn, Cd, and Pb(NO3)2
by cysteine alone [4]. Divalent ions of these metals complexed with
the cysteine sulfhydryl (-S- ) and amino groups. During the
adsorption process, the hydrogen of sulfhydryl was dissociated, accounting
for the pH drop. However, in the present case of AuCN2-
uptake, it was hard to dissociate Au from the cyanide complex at room temperature.
The present Au cysteine-aided biosorption probably still involved anionic
AuCN2- complex adsorption. A similar general behavior
was reported for biosorption of anionic Cr(VI) [12], whereby lowering of
the equilibrium pH from neutral to acidic yielded an increase in Cr uptake
by Sargassum. This was also the case for AuCN2-
uptake by Bacillus, Pencillium and Sargassum biomass
without cysteine addition [1]. Basically, sulfhydryl and amino groups on
cysteine behaved like weak bases which could be protonated at low pH, the
same as those found in biomass. When cysteine is encountered in aqueous
solution, there exists a surface charge on ionizable functional groups.
As the concentration of protons is increased at pH 2.0, more and more weak
base groups either on cysteine or in biomass become protonated and many
acquire a net positive charge. These charged sites become available for
binding anionic AuCN2- . Meanwhile, some carboxyl
groups on cysteine may still be dissociated as the solution pH was higher
than the dissociation constant (pK=1.9) of the carboxyl group on cysteine.
This allows binding on biomass through the combination with some positively
charged biomass function groups. As biomass bound cysteine, the amount
of weak base groups of AuCN2-.on biomass increased
resulting in enhanced Au uptake.
 |
3.3. Ionic strength effect
Ionic strength of experimental solutions was changed
from 0.005 M to 0.15 M by adding NaNO3 at pH 2. During the process,
there was no cyanide released from the solution just as the case was in
the pH effect experiments, indicating that sodium nitrate can not assist
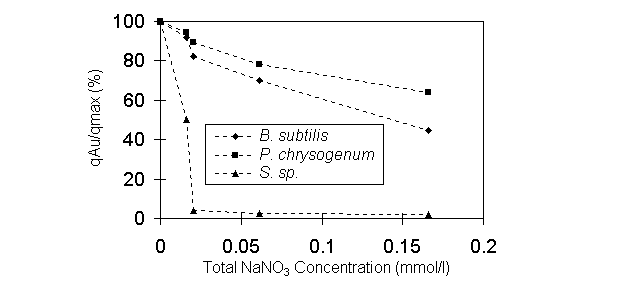
in dissociating the gold-cyanide complex. The effect of ionic strength
is illustrated in Figure 4. Increasing ionic strength reduced the Au biosorption.
As the concentration of NaNO3 increased to 68mM, the uptake
of Au by Bacillus and Penicillium biomass was respectively
reduced to 70% and 50% of that without NaNO3 in the solution.
The Au uptake by Sargassum decreased almost to zero at 20mM NaNO3.
Changing ionic strength (i.e. the background electrolyte concentration)
influences adsorption in at least two ways: (a) by affecting the interfacial
potential and hence the activity of electrolyte ions and adsorption; (b)
by affecting electrolyte ions and adsorbing anions competition for available
sorption sites.
 |
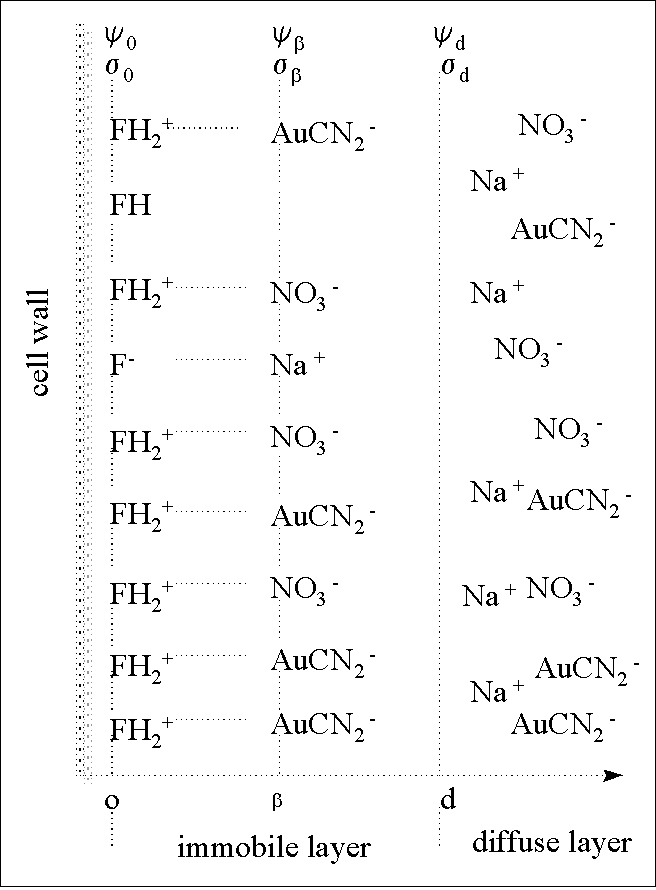 |
3.4. Desorption of Au-loaded biomass
The possibility of desorbing Au from biomass with
sodium hydroxide was examined by first sorbing Au onto biomass in the presence
of cysteine at pH 2 and then desorbing Au with 0.1 M NaOH at pH 3, 4 or
5. The initially Au-loaded Bacillus biomass contained 20.5 mmol
Au /g of dry biomass, Penicillium biomass 14.2 mmol/g,
and Sargassum biomass 4.7 mmol/g. The
percentage of Au recovery, represented by the ratio of the amount of Au
released per gram of the biosorbent during desorption and the equilibrium
sorption uptake, was calculated for desorption experiments lasting for
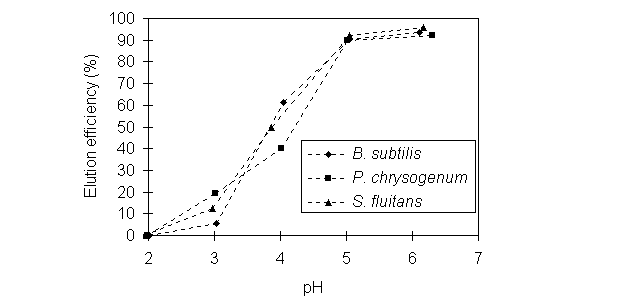
4 hours. Figure 6 shows that more than 90% of Au was recovered at pH 5
with the solid-liquid ratio S/L=4 for all of these three biomass types,
which indicated that Au could be easily eluted. These results further confirmed
the outer-sphere complexation (FH-H+-AuCN2-)
postulated. When the "bridge" was broken up by OH- combining with H+, Au
was dissociated from solid phase. Basically, the outer-sphere complex is
not stable probably because of the relatively long distance between sorbent
functional groups (on o-plane) and the sorbate (on b-plane). From the view
point of adsorption reaction equilibrium, increasing the pH pushed the
reaction of anionic gold cyanide complex adsorption to the left, allowing
the Au elution. However, in the case of biomass, the use of concentrated
NaOH leads to massive leaching of a variety of compounds from the biomass
and to the destruction of the biomass cellular structure. Therefore, Au
elution was limited to pH no higher than 6.
 |
F : functional group on biomass or cysteine
H: dissociable hydrogen on function group, the binding of which make
cysteine-loaded biomass neutral
so: surface charge on o-plane
sb : surface charge on
b-plane
sd : surface charge on d-plane
yo: potential on o-plane
yb: potential on b-plane.
REFERENCES
[1] Niu, H.; Volesky, B. 1999, J. Chem. Technol. Biotechnol.
(in press).
[2] Shindo, H.; Brown, T.L. J. Amer. Chem. Soc. 1965,
87, 1904-1908.
[3] Hussain, S.; Volet, B. In Vitro Toxicol. 1995, 8,
377-388.
[4] Li, N.C.; Manning, R.A. J. Amer. Chem. Soc. 1955,
77, 5225-5528.
[5] Percival, E.; McDowell, R.H. Chemistry and Enzymology of Marine
Algal
Polysaccharides,
Academic Press: London, U.K., 1967, pp 157-176.
[6] Marsden, J.; House, L. Chemistry of Gold Extraction, Ellis
Horwood: Hartnoll, UK, 1993,
pp 100-500.
[7] Riveros, P.A.; Molnar, R. CIM Bulletin 1996, 89,
153-156.
[8] Eaton, A.D.; Clesceri, L.S.; Greenberg, A.E. Standard Methods;
American Public Health
Association: Washington,
1995, pp 4.19 - 4.71.
[9] Perrin, D.D. Stability Constants of Metal-Ion Complexes,
Pergamon Press: Oxford, UK,
1979.
[10] Beveridge, T.J.; Murray, R.G.E. J.Bacteriol. 1976,
127, 1502.
[11] Troy, F.A.; Koffler, H. J. Biol. Chem. 1969, 244,
5563-5576.
[12] Kratochvil, D.; Volesky, B. Environ. Sci. Technol. 1998,
(in press).
[13] Fen, B.J.; Daughney, C.J.; Yee, N.; Davis, T.A. Geochim. Cosmochim.
Acta 1997, 61,
3319-28.
[14] Niu, H.; Xu, X.S.; Wang, J.H.; Volesky, B. Biotechnol. Bioeng.
1993, 42, 785-787.
[15] Kuyucak, N.; Volesky, B. Biorecovery 1989, 1,
189-204.
[16] Percival, E.; McDowell, R.H. Chemistry and Enzymology of Marine
Algal
Polysaccharides,
Academic Press: London, U.K., 1967, pp 83-98, 99-104, 127-156,
157-164.
[17] Schiewer, S.; Volesky, B. In Metals: Mine Drainage, Removal
and Toxicity ; Roddick,
F.; Britz, M.; Robbins,
N., Eds.; Kluwer: Dordrecht, The Netherlands, 1999 (in press).
[18] Kratochvil, D.; Fourest, E.; Volesky, B. Biotechnol. Lett.
1995, 17, 777-782.
[19] Hu, M.Z.-C.; Norman, J.M.; Faison, N.B.; Reeves, M. Biotechnol.
Bioeng. 1996, 51,
237-47.
[20] Huang, J.P.; Westman, J.; Quirk, K.; Huang, J.P. Water. Sci.
Technol. 1988, 20, 369-376. [21] Huang, C.-P.; Huang,
C.; Morehart, A.L. Water. Res. 1990, 24, 433-439.
[22] Yakubu, N.A. Ph.D. Thesis, University of London, U.K., 1986.
[23] Tien, C. Adsorption Calculation and Modeling, Butterworth:
Boston, 1994, pp 29-40.
[24] Rubin, A.J.; Mercer, D.L. Adsorption of Organics at Solid/liquid
Interface, Ann Arbor
Science: Ann Arbor, MI,
1981, pp 245.
[25] Hayes, K.F. J. Coll. Interf. Sci. 1988, 125,
717-726.
[26] Rothstein, A.; Meier, R. J.Cell.Comp.Physiol. 1951,
38, 245.
[27] Hayes, K.F. J. Coll. Interf. Sci. 1987, 115,
564-572.
[28] Marinsky, J.A. Ion Exchange, Marcel Dekker: New York, 1966,
2, pp 228-231.