BIOSORPTION OF Cu FROM FERRUGINOUS WASTEWATER
BY ALGAL BIOMASS 
Abbreviated version of a contribution shortly to be published in
WATER RESEARCH (1998)
David Kratochvil and Bohumil
Volesky
Department of Chemical Engineering, McGill University
3610 University Street, Montreal,
Quebec, Canada, H3A 2B2
ABSTRACT
The biosorbent prepared
from Sargassum algal biomass binds approximately 2.3 meq/g of metal
cations from water by ion exchange. The values of ion exchange equilibrium
constants showed that the affinities of metals towards the biosorbent decrease
in the following order Cu>Ca>Fe. A flow-through sorption column was used
to continuously and selectively remove Cu2+ from the feed containing
Cu and Fe ions. A chromatographic effect in the column performance caused
by different sorption affinities of the metal ions studied was successfully
predicted by the Equilibrium Column Model. The biosorbent saturated with
Cu was regenerated with 0.1M HCl. When Fe(III) was present in the mixed
feed solution as suspended solids (SS) the column removed Cu2+
by biosorption and Fe(III) solids by in-depth filtration while producing
effluent free of heavy metals from the feed containing 25 mg/L of Cu2+,
and Fe(III) as SS in the concentration range of 15-40 mg/L. Effective copper
removal/recovery from ferruginous wastewater using Sargassum biosorbent
was demonstrated.
INTRODUCTION
Usually, many types of aquatic
pollution caused by heavy metals also contain iron as one of the elements.
Recently, the biosorption equilibrium of a Cd-Fe system was studied and
a methodology allowing the evaluation of interferences in biosorption was
introduced (1). The main objective of this work was to investigate
the interference of iron in the biosorption of copper by Sargassum biosorbent,
while the second one was, to evaluate the alternatives of the biosorption
process which would selectively remove Cu from ferruginous wastewater ,
taking into account the speciation of iron in water.
IRON SPECIATION IN WATER
Iron typically enters bodies
of water in the form of ferrous iron (Fe2+) which can be oxidized
to ferric iron (Fe3+) by the oxygen dissolved in water according
to equation (1). The rate of the oxidation reaction (1) depends primarily
on the pH and on the level of dissolved oxygen (DO) in water. Consequently,
due to a lack of DO, Fe2+ is the predominant form of iron in
groundwater and in deep water reservoirs. At pH < 4 and a relatively
low DO, reaction (1) is very slow (2). At pH>4, however, Fe2+
ions oxidize quickly to Fe3+ ions which then react with water
according to equation (2) producing ferric hydroxide precipitate and acidity.
Fe2+ + 1/4 O2 + H+ = Fe3+
+ 1/2 H2O (1)
Fe3+ + 3 H2O = Fe(OH) 3 (s)
+ 3 H+
(2)
If the pH drops below 3, the
ferric ions cease to precipitate and remain in water in partially hydrolyzed
forms (3). In practice, the pH is usually raised above pH 3 by dilution
through a discharge of ferruginous effluents into streams and/or stabilization
ponds (3 , 4). It has been demonstrated that certain types
of Fe3+ precipitates can undergo a reductive dissolution in
the presence of organic matter according to equation (3) (5):
Fe3+(s) + soluble organics = Fe2+ + oxidized
soluble organics (3)
An example of an organic substance
reducing Fe3+ particles is fulvic acid (6).
In addition to iron and acidity, toxic heavy metals including Cu, Zn,
and Cd are often present in industrial effluents. The toxic heavy metals
do not react with dissolved forms of iron, however, their cations are capable
of sorbing onto the iron precipitate (7). A variety of conditions
including the water source, the pH, and the DO level, bring about a diversity
of effluents with metal content varying from traces to g/L levels (4).
THEORY OF BIOSORPTION
Recently, several researchers
have independently concluded that the major mechanism of heavy metal uptake
by algae (8 , 9), fungi (10), and peat moss (11)
is ion exchange. Furthermore, it has been demonstrated that algal bisorbents,
similar to ion exchange resins, can be prepared in different ionic forms
such as H-form and Ca-form (12). Consequently, ion exchange models
have been introduced to fit and interpret the data obtained from both equilibrium
and dynamic biosorption experiments (12,13). A binary ion
exchange system containing divalent metal ions A and B may be described
by the following exchange reaction
 (4)
The corresponding equilibrium constant is defined as
(4)
The corresponding equilibrium constant is defined as
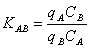 (5)
(Please consult the Symbols section at the end of
this text).
(5)
(Please consult the Symbols section at the end of
this text).
The value of the equilibrium
constant can be determined from the slope of the plot of versus . The overall
binding capacity Q (meq/g of dry biosorbent) is given by the density of
the functional groups in the sorbent and can be expressed as
Q = qA + qB
(6)
The total normality of the solution is given by
C0 + CA + CB
(7)
By substituting equation (6)
into (5), qB can be eliminated from (5) and the following
expression for (qA/Q) can be obtained:
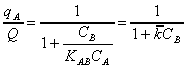 (8)
where
(8)
where 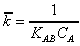 . Since (qA/Q) represents
the fraction of the binding sites occupied by A, equation (8) may be used
to evaluate the decrease of the equilibrium uptake of the species A by
the biosorbent due to the presence of species B. Equation (8) shows that
when CB = 0, (qA/Q)~ 1, regardless of the
absolute value of the final concentration of A, CA. This
distinguishes ion exchange from chemisorption and/or physical sorption
known to occur on activated carbon whereby (qA/Q) depends on
the value of CA. Furthermore, equation (8) shows that
for a fixed value of CA , the (qA/Q) is a
hyperbolic function of CB. Equation (8) may be transcribed
using the following dimensionless variables . Since (qA/Q) represents
the fraction of the binding sites occupied by A, equation (8) may be used
to evaluate the decrease of the equilibrium uptake of the species A by
the biosorbent due to the presence of species B. Equation (8) shows that
when CB = 0, (qA/Q)~ 1, regardless of the
absolute value of the final concentration of A, CA. This
distinguishes ion exchange from chemisorption and/or physical sorption
known to occur on activated carbon whereby (qA/Q) depends on
the value of CA. Furthermore, equation (8) shows that
for a fixed value of CA , the (qA/Q) is a
hyperbolic function of CB. Equation (8) may be transcribed
using the following dimensionless variables
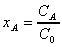 ; ; 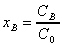 ; ; 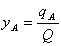 (9)
yielding equation (10) which represents the binary ion exchange isotherm
for the system
(9)
yielding equation (10) which represents the binary ion exchange isotherm
for the system
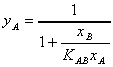 (10)
Since yA, yB, and KAB
are all dimensionless, equation (10) represents the most generalized description
of the equilibrium for binary systems.
(10)
Since yA, yB, and KAB
are all dimensionless, equation (10) represents the most generalized description
of the equilibrium for binary systems.
COLUMN FEED (methodology abbreviated)
Ca-saturated biomass of
Sargassum seaweed was packed in a 50 cm long column of 2.5 cm I.D.
yielding an approximate packing density of 200 g/L. Feed metal solutions
of FeSO4 and CuSO4 and Fe2(SO4)3
and CuSO4 were fed into the sorption column at pH 4 and at the
rate of 2 and 3 cm/min (0.5 and 0.75 gpm/ft2).
RESULTS AND DISCUSSION
According to equation (5),
the sorption equilibrium data for the two binary systems Fe-Ca and Fe-Cu
were plotted as (qA/qB) versus (CA/CB),
where A and B represented Fe and Ca, and Cu and Fe in the two plots, respectively.
Both plots yielded straight lines passing through the origin (Fe-Ca: y
= 0.3028 x; Fe-Cu: y = 6.7341 x), fitting the experimental data reasonably
well (Fe-Ca: R2 = 0.97; Fe-Cu: R2 = 0.99). This indicated
that the ion exchange model provides a relatively good description of the
biosorption of the heavy metals by the algal biomass. The values of ion
exchange equilibrium constants determined from the slopes of the above
mentioned straight lines were KFeCa = 0.3 and KCuFe
= 6.7 for the Fe-Ca and Fe-Cu systems, respectively. The equilibrium constants
defined by equation (5) are related to Gibbs free energy of ion exchange
reactions (4), and hence the values of the constants reflect the relative
affinities of the metals towards the binding groups in the Sargassum
biomass. Consequently, the metals can be sorted in the order of descending
affinity, based on the values of the equilibrium constants, as follows
: Cu>Ca>Fe.
Furthermore, once the equilibrium
constants KFeCa, and KCuFe are determined,
the ion exchange isotherms represented by equation (10) can be plotted
for the binary systems in question using the dimensionless coordinate system
[ yA , xA ] as shown in Figure 1. The
diagonal line in Figure 1 represents the hypothetical case of an equilibrium
where the composition of A in the liquid and in the sorbent are the same
thereby indicating that the sorbent is not selective for either of the
components of the binary system. Clearly, the sorbent is selective when
it sorbs preferentially either the species A or the species B from the
binary mixture. The former and the latter case are represented by equilibrium
isotherms of A which pass above and below the diagonal line, respectively,
in the [ yA , xA] coordinate system.
It can be seen from Figure 1 that both Cu and Ca are sorbed preferentially
from their respective binary mixtures with Fe over the entire concentration
interval. Furthermore, equation (5) shows that for equimolar binary mixtures
of Fe and Cu, i.e. when xFe = xCu =
0.5, the value of the equilibrium constant expresses the ratio of the metal
uptakes KCuFe = (qCu/qFe) . Hence, the
Sargassum biomass equilibrated with an equimolar mixture of Fe2+
and Cu2+ is expected to bind approximately 6.7 times more of
Cu than Fe.
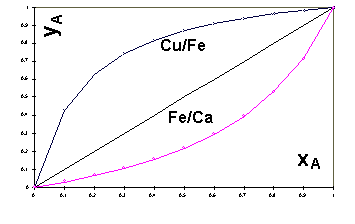 Figure 1: Binary ion exchange isotherms calculated from
Equation (10)
Figure 1: Binary ion exchange isotherms calculated from
Equation (10)
The natural selectivity of
the biomass for Cu over Fe is well reflected in the results obtained using
the flow-through biosorption column. Figure 2 displays the concentrations
of Fe2+ and Cu2+ in the column effluent as a function
of time for the sorption experiment during which the column packed with
Sargassum biosorbent in Ca-form was fed with an equimolar mixture
of Cu2+ and Fe2+. As can be seen in Figure 2, Fe2+,
due to its low affinity, broke through the column much faster than Cu2+.
At approximately the 35 bed volume mark, the concentration of Fe2+
in the column effluent plotted in Figure 2 reached the level of Fe2+
in the feed, i.e. (C/C0) = 1. Hence, thereafter, Fe2+
was no longer uptaken by the biosorbent and trickled down the packed-bed
as an inert.
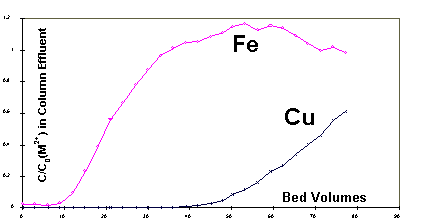 Figure 2: Biosorption column break-through curves for Fe
and Cu
Figure 2: Biosorption column break-through curves for Fe
and Cu
Exit Concentration Overshoot
Normally, when the breakthrough
of a column is complete, the exit concentration(s) will equal the feed
concentration(s), i.e. the column does not sorb any more, it is totally
saturated. The fact that C/C0 for Fe2+ continued
rising above 1 after 35 bed volumes in Figure 2 may be explained by the
ion exchange between Cu and Fe whereby Cu2+ from the solution
was displacing Fe already bound to the biosorbent. Since no more Fe2+
was being sorbed from the liquid beyond 35 bed volumes, the released Fe2+
increased the overall concentration of Fe2+ in the liquid above
the level present in the column influent. Different affinities of the two
metals cause the "chromatographic effect" seen at the column exit.
Tit is highly desirable
to predict the time and the magnitude of the exit concentration . This
can be done by using the EC Mathematical Model, by solving equations (5)
- (14), introduced earlier, for A, B, and C being Cu, Ca, and Fe, respectively.
This particular model has a limitation in its assumption of negligible
mass transfer resistance which explains why the model curve in Figure 2
is steeper than the experimental breakthrough curve for Fe2+
(in more detail in the full paper version)
A more sophisticated column
sorption model (14 ) used successfully in the recent continuous-flow
copper biosorption study (12) could accommodate the process mass
transfer aspects. However, the applicability of its present form is limited
to binary sorption systems. While its adaptation for three- and multi-component
sorption systems is possible, it requires the mass transfer coefficients
for the metal ions in the biosorption system. Such work is demonstrated
for Cd in the following pre-print posted here (Yang and Volesky, 1998).
Desorption
Desorption of the metals
deposited in the biosorbent is not only possible (12) but the determination
of its dynamics represents a crucial information for biosorption process
design and for metal recovery feasibility assessment. Figure 3 shows the
elution peaks of Cu and Fe obtained during desorption carried out with
0.1M HCl. The peak of Fe is much smaller than the peak of Cu which is in
agreement with the higher selectivity of the biomass for Cu. The desorption
of copper was approximately nine times faster than the process of the column
saturation.
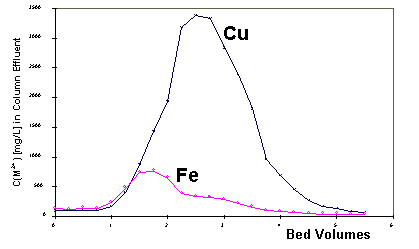 Figure 3: Desorption of heavy metals from Ca-biosorbent
by 0.1 M HCl
Figure 3: Desorption of heavy metals from Ca-biosorbent
by 0.1 M HCl
Effect of Fe on Cu biosorption
Owing to competitive sorption,
the uptake of a species from a multicomponent mixture is lower than the
uptake of the same species from the single component system. Figure 4 summarizes
the effect of the increasing concentration of Fe2+ on the uptake
of Cu by Sargassum biosorbent. The curves in Figure 4, calculated
using equation (5), show the equilibrium uptake of Cu expressed as the
fraction of the maximum Cu uptake plotted against the equilibrium concentration
of Fe2+ in the liquid at equilibrium Cu2+ concentrations
of 0.25, 1, and 4 meq/L (8, 33, and 120 mg/L). The lower the Cu concentration
in the liquid, the greater the overall reduction of Cu uptake caused by
Fe2+, and the lower the amount of Fe2+ needed to
decrease the Cu uptake by a fixed percentage.
Ferruginous wastewater with
a relatively high level of DO and a pH > 3 is likely to contain most of
the iron in the form of Fe3+ suspended solids (SS). The biosorption
column can effectively sorb Cu from the feed while functioning as a filter
for the Fe3+ solids at the SS level of 15 and 40 mg/L. Furthermore,
a linear pressure drop build-up due to the in-depth retention of solids
into the packed-bed (15) was relatively slow, hence allowing the
operation of the column to continue until the breakthrough of Cu. However,
the Cu breakthrough occurred earlier as the level of SS in the feed increased
due to the physical blockage of sorption sites in the biomass. The fact
that the pressure drop across the column increased approximately linearly
with time provides an indication that most of the solids penetrated deeply
into the bed.
As long as all of the iron
present in the water is in the form of either Fe3+ SS, or Fe2+,
the oxidation of the biosorbent by Fe3+ is prevented. The reductive
dissolution of Fe3+ SS by soluble organic matter leaching from
the biosorbent may occur. However, the dissolution of the Fe3+
SS would not substantially interfere with Cu removal considering the time
scale of one biosorption cycle and the fact that the dissolution according
to equation (3) is known to be a very slow process (5, 6
).
CONCLUSIONS
The options for a biosorption
process utilizing Sargassum biosorbent to remove and recover copper
from ferruginous water are summarized below for the two extreme cases of
water containing only ferrous form iron and water containing exclusively
ferric form of iron.
I. Water with a low level of DO and all of the iron in ferrous (Fe2+)
form
Using [C(Cu2+),
C(Fe2+)] coordinates, the regions of high, intermediate, and
negligible interference of Fe2+ in the sorption of Cu2+
were constructed in Figure 5. In the regions of high and negligible interference,
iron lowers the uptake of copper by more than 40%, and by less than 5%,
respectively, as compared to the copper uptake from water containing only
Cu2+. The borders between the regions in Figure 5 were calculated
using equation (8) and fixed values of q(Cu)/Q of 0.6 and 0.95, respectively.
It is clear that the removal /recovery of Cu from ferruginous water by
biosorption can be considered effective only if the metal content of water
falls into the ranges of either low or intermediate interference.
II. Water containing only ferric iron in the form of Fe(III) suspended
solids
Figure 6 summarizes the
interference of Fe3+ SS in the removal of Cu2+ by
biosorption. Water containing less than 40 mg/L Fe3+ SS may
be directly fed into biosorption columns. If the level of SS in the water
exceeds 40 mg/L, a partial removal of SS in a pond and/or a clarifier is
necessary prior to biosorption of copper in columns. Depending on Cu/Fe
molar ratio and pH, however, a portion of the overall Cu content may sorb
on the Fe3+ precipitate during settling. The lines indicating
the percentage of the overall Cu content sorbing on Fe3+ SS
in Figure 6 were calculated from data previously published by Gutzman et
al. (7 )
SYMBOLS
A, B divalent metal species
C(M) concentration of metal M in the liquid
[ meq/L ]
C0(M) concentration of metal M in
the feed to a flow-through column [meq/L]
C(M)/C0(M) relative concentration
in column effluent [-]
KAB equilibrium ion exchange constant
defined by equation (5) [-]
q(M) equilibrium uptake of metal M by the biosorbent
[ meq/g ]
Q concentration of
metal binding sites in the biosorbent [ meq/g ]
xA equilibrium
equivalent fraction of species A in the liquid [-]
yA equilibrium
equivalent fraction of species B in the sorbent [-]
T dimensionless
time in the ECM model [-]
REFERENCES
(1) Figueira, M.M.; Volesky, B.; Ciminelli, V.S.T. Biotechnol. Bioeng.
1997,
54, 344-350.
(2) Stumm, W.; Morgan, J.J. Aquatic Chemistry, Wiley Interscience:
New York, 1981, pp
779-781.
(3) Dudeney, A.W.L.; Ball, S.; Monhemius, A.J. Treatment Processes
for Ferruginous
Discharges
from Disused Coal Workings, R&D Note 243, National River
Authority: Bristol,UK,
1994.
(4) Gazea, B.; Adam, K.; Kontopoulos, A. Minerals Engineering
1996, 9, 23-42.
(5) Vile, M.A.; Wieder, K.R. Water, Air, and Soil Pollution
1993, 69, 425-441.
(6) Deng, Y.; Stumm, W. Aquatic Sciences 1993, 55,
1015-1621.
(7) Gutzman, D.W.; Langford, C.H. Environ. Sci. Technol. 1993,
27, 1388-1393.
(8) Crist, R.H.; Martin, J.R.; Guptill, P.W.; Eslinger, J.M.; Crist,
D.R.
Environ.
Sci. Technol. 1990, 24, 337-342.
(9) Kratochvil, D.; Fourest, E.; Volesky, B. Biotechnol. Lett.
1995, 17, 777-782.
(10) Fourest, E.; Roux, J.C. FEMS Microbiol. Rev. 1994,
14, 325-332.
(11) Spinti, M.; Zhuang, H.; Trujillo, E.M. Water Environ. Res.
1995, 67, 943-952.
(12) Kratochvil, D.; Volesky, B.; Demopoulos, G. Water Res.
1997, 31, 2327-2339.
(13) Schiewer, S.; Volesky, B. Environ. Sci. Technol. 1997,
31, 2478-2485.
(14) Tan, H.K.S.; Spinner, I.H. Can. J. Chem. Eng. 1994,
72, 330-341.
(15) Cleasby, J.L.; Baumann, E.R. Jour. AWWA 1962, 54,
579-592.
[ Bact to the Beginning ]
[ Bact to the Index ]
[ Dr. Volesky's
Home ] |
 (4)
(4)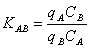 (5)
(5)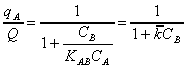 (8)
(8)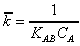 . Since (qA/Q) represents
the fraction of the binding sites occupied by A, equation (8) may be used
to evaluate the decrease of the equilibrium uptake of the species A by
the biosorbent due to the presence of species B. Equation (8) shows that
when CB = 0, (qA/Q)~ 1, regardless of the
absolute value of the final concentration of A, CA. This
distinguishes ion exchange from chemisorption and/or physical sorption
known to occur on activated carbon whereby (qA/Q) depends on
the value of CA. Furthermore, equation (8) shows that
for a fixed value of CA , the (qA/Q) is a
hyperbolic function of CB. Equation (8) may be transcribed
using the following dimensionless variables
. Since (qA/Q) represents
the fraction of the binding sites occupied by A, equation (8) may be used
to evaluate the decrease of the equilibrium uptake of the species A by
the biosorbent due to the presence of species B. Equation (8) shows that
when CB = 0, (qA/Q)~ 1, regardless of the
absolute value of the final concentration of A, CA. This
distinguishes ion exchange from chemisorption and/or physical sorption
known to occur on activated carbon whereby (qA/Q) depends on
the value of CA. Furthermore, equation (8) shows that
for a fixed value of CA , the (qA/Q) is a
hyperbolic function of CB. Equation (8) may be transcribed
using the following dimensionless variables
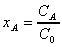 ;
; 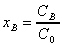 ;
; 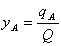 (9)
(9)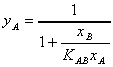 (10)
(10)

